Thermodynamics
Basic Calorimetry Set
Basic Calorimetry SetUses an inexpensive styrene foam calorimeter, five different metal samples, and more to allow students to investigate the specific heat of substances, the latent heats of vaporization and fusion of water.
Blackbody Radiation Experiment
Blackbody Radiation ExperimentThe complete solution for demonstrating the blackbody spectrum of light intensity for a light bulb. Great for qualitative analysis.
Calorimeter with isolating styrofoam
Calorimeter with isolating styrofoamThis calorimeter can e.g. used together with FR-274010 for verification of Joule’s law and FR-274020 for investigation of energy conservation.
The calorimeter consists of an inner cup and an outer cup separated by an insulating layer of flamingo. The cups are made of aluminum with a beaded edge and a polished exterior.
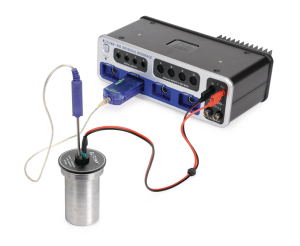
Electrical Equivalent of Heat – Wireless
Electrical Equivalent of Heat – WirelessA Wireless Current Sensor and a Wireless Voltage Sensor are used to measure the electrical power delivered to the resistor that is heating the water and a Wireless Temperature Sensor is used to measure the increase in temperature of the water.
Electrical Equivalent of Heat Experiment
Electrical Equivalent of Heat ExperimentThe complete solution for investigating the relationship between mechanical work and heat. This complete solution is designed for use with PASCO Capstone Software.
Energy Meter
Energy MeterThe Lascells Energy Meter provides an all-in-one solution to teaching Specific Heat Capacity. With real-time read out of voltage, current, time and temperature, all measurement parameters are provided for students to calculate Specific Heat Capacity from first principles.
Energy Transfer – Calorimeter
Energy Transfer – CalorimeterThis unique calorimeter uses a heating resistor instead of a spring coil to heat a small amount of water.
Heat Engine Cycle – Wireless
Heat Engine Cycle – WirelessA P-V diagram is generated as a heat engine is taken through a cycle. From this diagram, the heat added to the gas and the work done by the engine are measured to determine the efficiency of the engine. This actual efficiency is compared to the theoretical maximum efficiency.
Heat Engine Cycles Experiment
Heat Engine Cycles ExperimentThe complete solution for examining the efficiency of heat engines and compared to the ideal gas law.
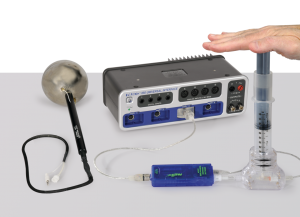
Ideal Gas Law – Wireless
Ideal Gas Law – WirelessIn this experiment designed for use with PASCO Capstone software, the temperature, volume, and pressure of a gas are measured simultaneously to show that they change according to the Ideal Gas Law.
Ideal Gas Law Experiment
Ideal Gas Law ExperimentThe complete solution for investigating the Ideal Gas Law, including the special cases of Boyle’s Law and Gay-Lussac’s Law.
Leslie’s Cube – Thermal Radiation Cube – COMING SOON
Leslie’s Cube – Thermal Radiation Cube – COMING SOONLeslie’s Cube – Thermal Radiation Cube – COMING SOON
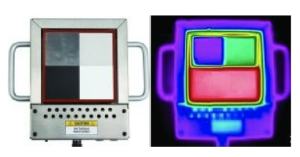
Leslie’s Cube – Thermal Radiation Cube – COMING SOONDemonstrate thermal radiation from four different surfaces. The aluminium plate heats to a uniform temperature of approximately 70°C or 100°C, depending on the setting. The four surfaces (black, white, silver, and polished silver) have different thermal emissivities, causing them to radiate different amounts of radiation even though they are all the same temperature. The photo shows the Leslie’s Cube in visible light on the left and in the infrared on the right.
Lid for calorimeter
Lid for calorimeterThis lid is used together with calorimeter FR-274050, and can be used for e.g. experiment with energy conservation.
Supplied with plug for hole suitable for Pasco sensor or a plug with hole suitable for stick thermometer.
Radiation Cans
Radiation CansThree cans in different colors to quantitatively investigating a full range of heat transfer phenomena.
Ratio of Specific Heat Experiment
Ratio of Specific Heat ExperimentThe complete solution for determining the ratio of specific heat capacities for air by measuring the period of oscillation of the piston in a cylinder filled with air.
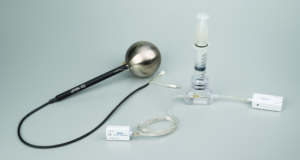
Ratio of Specific Heats – Wireless
Ratio of Specific Heats – WirelessIn this experiment, the ratio of specific heat capacities for air is determined using Ruchardt’s method of measuring the period of oscillation of the piston in a cylinder filled with air.
Specific Heat – Wireless
Specific Heat – WirelessIn this experiment designed for use with PASCO Capstone software, students learn that materials can be identified using specific heat as a measurable characteristic. A known mass of water is used and the unknown material is placed in the water. The initial temperature of the water and the unknown material are measured. The equilibrium temperature is found and from this the specific heat of the unknown material is derived.
Specific Heat Experiment
Specific Heat ExperimentStudent experiment to identify materials using specific heat as measurable characteristic. This complete solution is designed for use with PASCO Capstone Software.
Steam Generator
Steam GeneratorGenerates steam for thermal expansion and heat conduction experiments